The CDC issued new guidance about the COVID-19 vaccine for people with underlying medical conditions
The CDC issued new guidance about the COVID-19 vaccine for people with underlying medical conditions
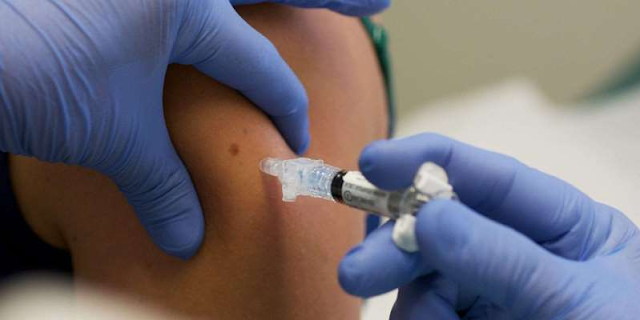
- The Centers for Disease Control and Prevention (CDC) issued new guidance on Saturday for those with underlying medical conditions, saying they can receive the COVID-19 vaccine as long as they "have not had a severe allergic reaction to any of the ingredients in the vaccine."
- The CDC specifically issued guidance for those with HIV, weakened immune systems, and autoimmune conditions like Guillain-Barre syndrome and Bell's palsy.
- Nearly 2 million people in the US have received the first of two doses of the vaccine as of Saturday, according to the CDC, but it still urges people to follow health guidelines.
The Centers for Disease Control and Prevention issued new vaccine guidance on Saturday for people with underlying medical conditions.
The CDC said that people with underlying medical conditions are at an increased risk of "severe illness" from the coronavirus and can get the COVID-19 vaccine as long as they "have not had a severe allergic reaction to any of the ingredients in the vaccine."
The CDC specifically issued guidance for people with the following conditions:
- HIV and weakened immune systems
- Guillain-Barre syndrome (GBS), which is a "rare, autoimmune disorder in which a person's own immune system damages the nerves," according to the CDC.
- Bell's palsy, which causes sudden and temporary weakness in facial muscles.
- Other autoimmune conditions
The CDC warned that information for the safety of the vaccine for those with HIV and weakened immune systems isn't available yet and while people living with HIV were included in clinical trials of the vaccine, data for that group isn't available either.
People who have had GBS and Bell's palsy can receive the vaccine, the CDC said.
"With few exceptions, the independent Advisory Committee on Immunization Practices (ACIP) general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines," the CDC said.
The CDC said that cases of Bell's palsy were reported in participants in the vaccine clinical trials, but the Food and Drug Administration has "not concluded these cases were caused by vaccination."
As of Saturday, 1.94 million people in the US have received the first of two doses of the vaccine, according to the CDC. But the agency still urges people to follow proper health guidelines such as wearing a mask and social distancing, even for those who receive the vaccine.



All BJP WORKER'S & NETA'S 1st INJECTION !
ReplyDeleteMP/MLA/Zilla/Pancha Garm/...... Bajrang & Sangha Parivar / VHP Mamata too !!!!
ReplyDelete1st dose to farukh Abdullah,Omar abdullah ,mehbooba mufti Salim chagla,Ali Shah Gilani, unseen malik etc.
DeleteWhy not 1st dose to Salim chagla,farukh and Omar Abdullah,mehbooba mufti,yaseen malik ali shah Gilani,manta bano etc.
DeleteHealth Care is always without any discrimination . I admire the efforts of our Prime Minister in Health Care System . Long Live Modiji .
ReplyDelete